Eliminating elemental mercury
Mercury is a naturally occurring trace element that is found in almost all fossil fuels. The predominant species found in natural gas and natural gas liquids (NGL) is elemental mercury. Low levels of colloidal and ionic species may also be found in streams within gas processing facilities. Colloidal mercury is insoluble as it is mercury bound to particulate matter, and ionic mercury is soluble mercuric salt, such as mercuric chloride.1
The concentration of mercury found in natural gas varies widely from ng Hg/Nm3 in some parts of the US and Africa, up to thousands of µg/Nm3 in some fields in North Germany.2 Reported levels of mercury found in natural gas are shown for different regions in Table 1.
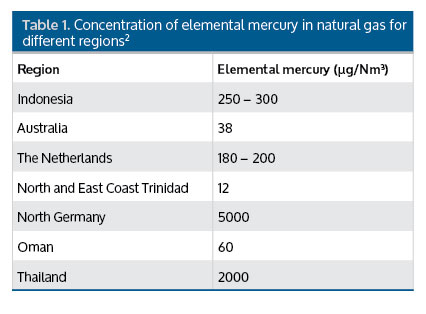
Table 1. Concentration of elemental mercury in natural gas for different regions.2
Due to the volumes of natural gas processed and the tonnages of liquid hydrocarbons handled, the quantities of mercury available to distribute throughout gas processing facilities can be substantial. A typical 10 000 tes/d LNG plant would use approximately 600 million ft3/d of natural gas. If this contained 100 µg/Nm3 of mercury, the plant would receive 582 kg/yr of mercury.2
Why remove mercury?
There are multiple issues associated with processing mercury-containing hydrocarbons. Key reasons for removing mercury include:
- Corrosion of process equipment.
- Exposure to personnel during maintenance operations.
- Difficulty in disposal of mercury-contaminated equipment.
- Emissions to the environment.
Corrosion
Corrosion of aluminium heat exchangers is a key concern for LNG plants, especially following the mercury induced catastrophic failure of a heat exchanger at Skikda, Algeria, in 1957. Mercury can corrode aluminium via the mechanism of either amalgam or liquid metal embrittlement (LME).
Amalgam corrosion is caused when the protective oxide layer on aluminium is breached, such as the surface being scratched during maintenance activities, and the metal is then exposed to mercury, water, and oxygen. The reaction takes place rapidly, with a subsequent reduction in structural integrity. LME corrosion simply requires mercury to diffuse through grain boundaries within the aluminium metal structure. This occurs rapidly, forming cracks and weakening the structure.
In order to avoid mercury induced corrosion of aluminium, removal of mercury to an upper limit 0.01 µg/Nm3 upstream of liquefaction is set in order to maintain equipment integrity within LNG plants.3
References
- CARNELL, P. J. H., ROW, V. A., and MCKENNA, R., ‘A Re-Think of the Mercury Removal Problem for LNG Plants’, (2008), pp. 1 – 8.
- CARNELL, P. J. H., JOSLIN, K. W., and WOODHAM, P., ‘Fixed-Bed Processes Provide Flexibility for COS and H2S Removal’, 74th Annual GPA Conference, San Antonio, Texas, US, (1995).
- CARNELL, P. J. H., ROW, V. A., and MCKENNA, R., ‘Minimising Mercury Emissions from Gas Processing and LNG Plants’, Hydrocarbon World, (2007).
This article was originally published in the May issue of LNG Industry magazine. To read the full version of this article, please sign in or register for a free trial subscription.
Written by Heather Whittenbury, Johnson Matthey, UK
For more information, click here.
Read the article online at: https://www.lngindustry.com/special-reports/09052016/eliminating-elemental-mercury-4587/
You might also like
Star of the Seas joins Royal Caribbean fleet
Royal Caribbean has welcomed another LNG-powered cruise ship, the Star of the Seas, to its fleet.

